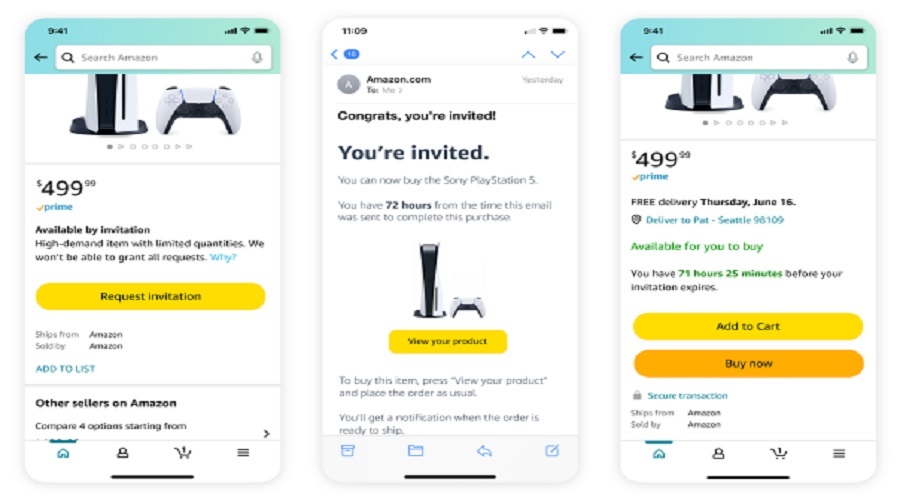
A Ban Imposed by FDA on Antibacterial Soaps Using Specific Chemicals
The U.S FDA (Food and Drug Administration) has announced in the month of September that the manufacturers of OTC (over-the-counter) consumer antiseptic was products having more than 19 specific active elements such as triclocarban and triclosan will not be permitted to sell their products. The FDA issued a statement that companies manufacturing those products, but unable to provide proves that their added specific elements are safe or more efficient than ordinary available water and soap in order to stop spread of infection and disease. The statement of FDA mentioned that it is not a science-rocket to give evidence that OTC antibacterial soaps are more effective in stopping disease instead of using a plain soap and water.
Point to be noted that the benefits haven’t yet been proven of using antibacterial hand soap. The excessive use of these products also raised questions about possible negative effects for the skin. The Director of CDER (Center for Drug Evaluation & Research) of FDA said that most of the consumers believe that antibacterial products are more effective and avoiding to spread disease-causing germs, but we are unable to find any scientific evidence claiming that those washing products are more efficient in its functionality instead of using regular soap & water. At least 40 percent of antiseptic hand soaps have chemicals such as triclosan, and it is being used at least 92 percent in antibacterial & antimicrobial liquid products. It is important that Triclosan was first used in hospitals in 1960s in order to kill microorganisms. The recent lab tests have indicated that bacteria have become resistant to triclosan and its wide use can cause critical problem of antibiotic resistance.