Pfizer to announce Single-pill Treatment for Covid-19

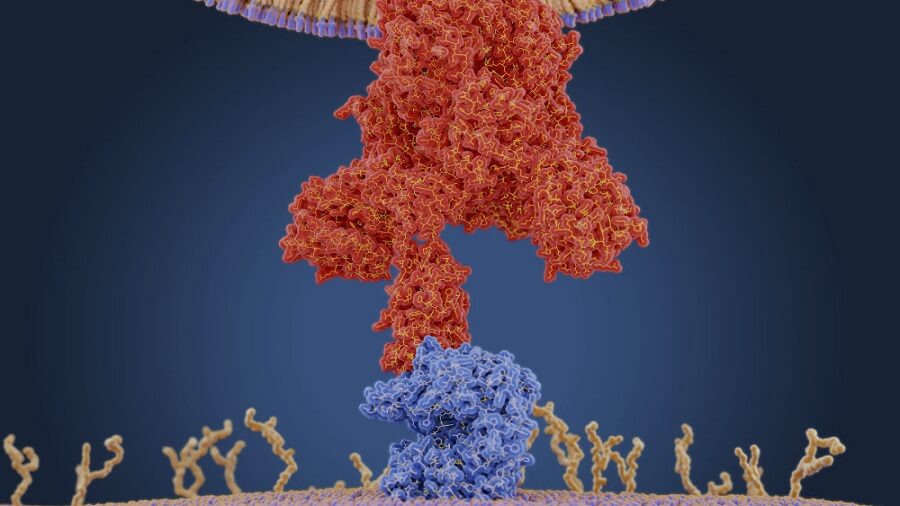
Pfizer is a famous drug manufacturer and currently testing a single-pill treatment for COVID-19. The drug will be available during the current year after its final trials. The new drug will be called PF-07321332, but it is currently in a Phase One clinical trial with healthy adults. The Telegraph also reported that the protease inhibitor may be available as soon as this year. The pill was announced at the American Chemical Society Spring 2021 meeting in early April. The new drug efficiently works by targeting the main protease of SARS-CoV-2 (the virus that causes COVID-19). The drug would prevent the virus from reproducing itself within the body. The chief science officer of Pfizer, Mikael Dolsten issued a press release and said that the pill could be prescribed at the first sign of infection without requiring critical care or hospitalization.
The head of medicine design of Pfizer, Charlotte Allerton said, “For the foreseeable future, we will expect to see continued outbreaks from COVID-19. And therefore, as with all viral pandemics, it’s important we have a full toolbox on how to address it”. It is noteworthy that protease inhibitors are currently used to treat HIV around the world. Pfizer’s chairman and CEO Albert Bourla earlier issued a statement about its Covid-19 vaccine. He said the recent data confirms the favorable efficacy and safety profile of our vaccine and can permit the company to apply for full approval from the US Food and Drug Administration. Currently, the vaccine only has Emergency Authorization Use, which was issued in December of 2020. He added the newest data confirms that the mRNA vaccine is 95.3% effective against severe COVID-19 as defined by the FDA and 100% effective against what the CDC defines as severe disease.
There have been more than 44,000 participants in total throughout the study of the vaccine’s safety. The company said 12,000 participants have followed up after their second dose to assure that the vaccine is effective for up to 6 months. The study also found that the vaccine was 100% effective against the variant that was first discovered in South Africa. Moreover, that study into the B1.351 variant only involved 800 participants. The CEO and co-founder of BioNTech, Ugur Sahin said, “It is an important step to further confirm the strong efficacy and good safety data we have seen so far, especially in a longer-term follow-up. These data also provide the first clinical results that a vaccine can effectively protect against currently circulating variants, a critical factor to reach herd immunity and end this pandemic for the global population”.