Covid-19 Vaccine distribution will be protected by the US Marshals

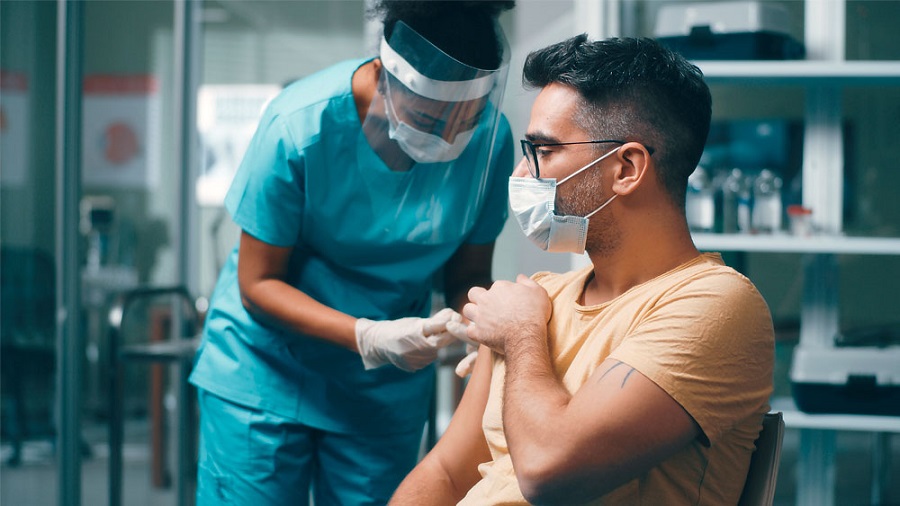
On Thursday, the US Marshals said it would protect a COVID-19 vaccine as it travels throughout the United States. The news came as the FDA (Food and Drug Administration) is expected to approve Pfizer’s vaccine for emergency authorization. The US Marshals issued a statement and said, “Deputy Marshals are working hand-in-hand with Operation Warp Speed personnel to provide security for COVID-19 vaccines from the facilities where they are manufactured to distribution sites”. Point to be noted that the Strategic National Stockpile Security Operations Program indicated that deputies will be stationed at several points during the distribution process to protect storage stockpiles, manufacturers, transports, and receiving facilities. The agency will be responsible for disseminating medical materials and pharmaceuticals to the American people in a time of crisis.
On Thursday, a federal advisory panel has also recommended Pfizer’s vaccine for approval. It would trigger a nationwide operation to ship 3 million doses of the vaccine across the country if the FDA approves the drug. The UPS started shipping vaccine kits, which arrived at some hospitals on Thursday. These kits contain syringes, masks, and a diluting agent for the Pfizer vaccine, as it will be used to administer the drug. The recent clinical trials showed the Pfizer vaccine was around 95% effective for adults 18 to 64 and was just as effective for people of all ethnicities. Some groups including people with weak immune systems, individuals with severe allergic reactions, and pregnant women could be restricted from getting the shot.
It is noteworthy that the US recorded its highest single-day death count, with over 3,000. Nearly 300,000 Americans have died from the virus. The news came as the widespread Covid-19 has also infected more than 15 million Americans. The United States is focused on getting 40 million vaccine doses of both Pfizer and Moderna Inc. Some state officials have said they anticipate prioritizing people at greater risk of contracting the disease, including elderly people and healthcare workers. It is important that the White House has planned the vaccine must be given twice for full effectiveness by the end of the year.